 Case Report
Case Report
CKD-Mineral Bone Disease, A Feature of Delayed Diagnosis of Congenital Anomaly of The Kidney and Urinary Tract in An Eight-Year-Old Nigerian Boy- Case Report
Adebukola Ajite1,2*, Oludare Oluwayemi1,2, Boluwatife Oniyide2 and Samuel Adisa2
1Ekiti State University, College of Medicine, Department of Paediatrics and Child health, Ado Ekiti, Nigeria
2Ekiti State University Teaching Hospital, Department of Paediatrics, Ado Ekiti, Nigeria
Adebukola Ajite, Ekiti State University, College of Medicine, Department of Paediatrics and Child health, Ado Ekiti, Nigeria
Received Date:March 22, 2024; Published Date:March 27, 2024
Abstract
Delay in identifying congenital anomaly of the kidneys and urinary tract (CAKUT) is often encountered in developing countries. This is majorly from poor health seeking behavior and unawareness of the relevance of non-invasive investigation tools that may aid early diagnosis and prompt management of such before progression to chronic kidney disease (CKD) Mineral Bone Disease and End State Renal Disease (ESRD). This is a case report of an 8-year-old boy who presented with difficulty in breathing of one week duration and fever of 12 hours duration. There were no oedema, oliguria, or hematuria. He had a previous medical history of being managed for multiple deformities of the limbs and suspected rickets 6years prior to index presentation but he defaulted. He was noticed to be small for his age, had rachitic rosary, pectus carinatum, double malleoli sign in both ankles and was anemic with packed cell volume of 14.9%, the serum creatinine and urea were elevated (323.3μmol/l and 20.1mmol/l respectively), there was hypocalcemia(1.10mmol/l), elevated alkaline phosphatase (263 IU/l). Abdominopelvic ultrasound showed absent left kidney and right polycystic kidney disease. Sedimented red blood cell was transfused, antibiotic, oral calcium and vitamin D were given, and he was referred for preemptive preparation for renal replacement therapy.
Keywords: CKD-mineral bone disease; end stage renal disease; congenital anomaly of the kidneys and the urinary tract; renal replacement therapy
Introduction
CKD-mineral bone disease (MBD) is a term coined by the kidney disease-Improving Global Outcomes (KDIGO) Foundation to describe the wide spectrum of systemic disorder of mineral and bone metabolism due to chronic kidney disease (CKD) [1]. Chronic kidney disease (CKD) is the presence of kidney damage (structural or functional abnormality involving pathological, laboratory, or imaging findings) for > 3 months or a glomerular filtration rate (GFR) < 60 ml/min/1.73 m2 for > 3 months [2].CAKUT contribute to the burden of CKD in the developing world as there is delay in diagnosis and barriers to management such as; late presentation, poor socioeconomic conditions, absence of medical insurance, limited diagnostic facilities and non-availability and affordability of chronic renal replacement therapy (RRT) [3-7]. It is imperative to increase awareness of the relevance of non-invasive investigations such as ultrasound done in pregnancy and perinatal period as useful tools in identifying children with congenital anomalies inutero so that prompt interventions, delivery and management can be planned.
Case Report
We present an 8-year-old boy whose admission to our health care facility was premised on the following: Presenting complaints were difficulty with breathing of one week duration and fever of 12 hours prior to presentation. Difficulty with breathing was associated with fast breathing which was insidious in onset, no relieving or aggravating factors and became progressively worse. There was no bluish discoloration of lips or fingers, no history of cough, noisy breathing, body swelling or reduction in urine output. He became febrile 12 hours prior to presentation, fever was on most times and was only temporarily relieved with oral paracetamol.
There was a background history of deformity of the limbs first noticed when he was 2years old, he subsequently presented at a tertiary health care facility where he was investigated for “rickets”. He was commenced on tablets vitamin D and vitamin C. However, patients defaulted from clinic visits and discontinued medications 4years ago. Urine volume was noticed to be usually much by the mother; she attributed this to the fact that the child was drinking much water.
There was no history suggestive of hematuria. Mother
had antenatal care at a primary health care facility and no
abdominopelvic ultrasound was done in pregnancy. He had delay
in walking, though he started crawling at 7month, he did not
walk until the age of 2years. He was the second of three children
in a monogamous family; siblings were alive and apparently well.
The parents were petty traders, and their highest level of formal
education was secondary school.
a) General physical examination: He was conscious, small
for age, in obvious respiratory distress (flaring ala nasi and
intercostal recession), febrile (39oC), pale, anicteric, acyanosed,
no evidence of dehydration, had mild facial puffiness but no
pedal edema.
b) Anthropometry: Weight was 16Kg (62.8% of the expected
for his age), Occipitofrontal circumference was 49.8cm, the
apparent length was 86cm (67.2% of the expected).
Systemic examination
a) Respiratory: Respiratory rate- 56ycles/minute, pectus
carinatum, rachitic rosary bilaterally Trachea was centrally
located, chest expansion was equal on both sides. Percussion
note was resonant. The breathing sound was vesicular. The
SPO2 was 88% in room air, 92% on intranasal oxygen.
b) Cardiovascular- Pulse rate was 110beats/min, regular,
full volume. Apex beat was at the 5th left intercostal space midclavicular
line. Blood pressure was 130/70mmHg. The first and
the second heart sound were heard, there was no murmur.
c) Gastrointestinal: Abdomen was full, moved with
respiration, there was no area of tenderness. The liver and
spleen were not palpably enlarged while the right kidney was
bimanually palpable. Bowel sounds were normoactive.
d) Genito-urinary: There was circumcised male external
genitalia with two testes palpable par scrotum. The urethra
meatus was of normal size.
e) Musculoskeletal: There was posterior deviation of distal
ulnar, bilaterally, presence of bilateral tibia sabre and double
malleoli sign on the ankles
f) Problems Identified: Breathlessness, tachypnea, anaemia,
growth failure, features of rickets, high blood pressure.
Investigations
Abdominopelvic ultrasound- the right kidney was significantly enlarged measuring 15.5cm×8.7 cm and it was completely replaced by multiple cystic lesions with sizes ranging between 3.4-12.5 cm. The cysts have thin walls and contain anaechoic fluid. No normal renal parenchyma tissue was seen. The left kidney was not seen, no evidence of ectopic kidney, urinary bladder was mildly filled with fluid. No cysts demonstrable in other abdominal organs whose dimensions were within normal limits. The final impression was, absent left kidney (renal agenesis) and right polycystic kidney disease.
Results
Blood electrolyte, urea, and creatinine.
Na---137.5mmol/L, K---4.9 mmol/L, CL---112 mmol/L.
HCO3---19mmol/L, Phosphate---0.84mmol/L.
Urea---20.1 mmol/L, Cr---323.3 μmol/L.
Serum Calcium – 1.10 mmol/L.
Hb genotype was AA, retroviral screening was non-reactive.
Full blood count; WBC -9.66x109/L, Lymphocyte – 18.08%.
Neutrophil- 69.32%, Hct – 14.9%, Platelet – 215X109/L.
Urinalysis- pH was 5.0, nil hematuria or proteinuria.
Liver function test (LFT) – Total bilirubin – 7.6mmol/L,
Conjugated bilirubin – 4.7mmol/L.
Total serum Protein- 70g/L, Albumin- 41g/L, AST- 16IU/L, ALT-
0.6IU/L, ALP- 263IU/L.
Picture and X-ray of the child are as shown below (Figures 1-4).
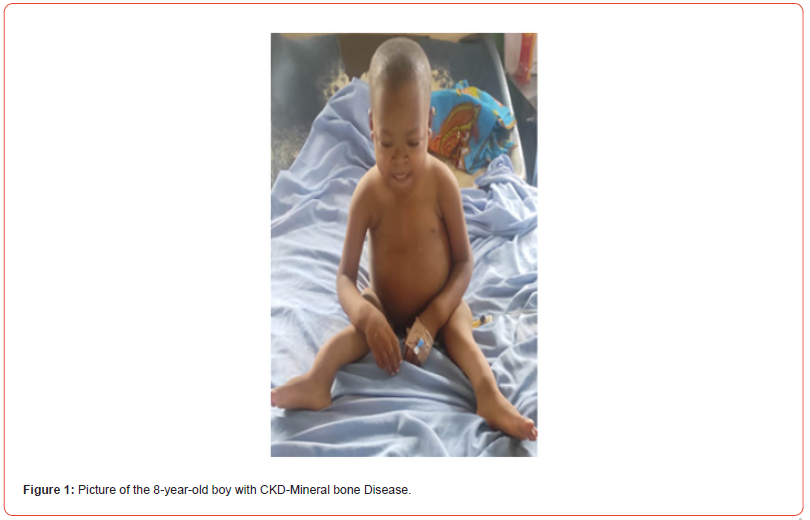
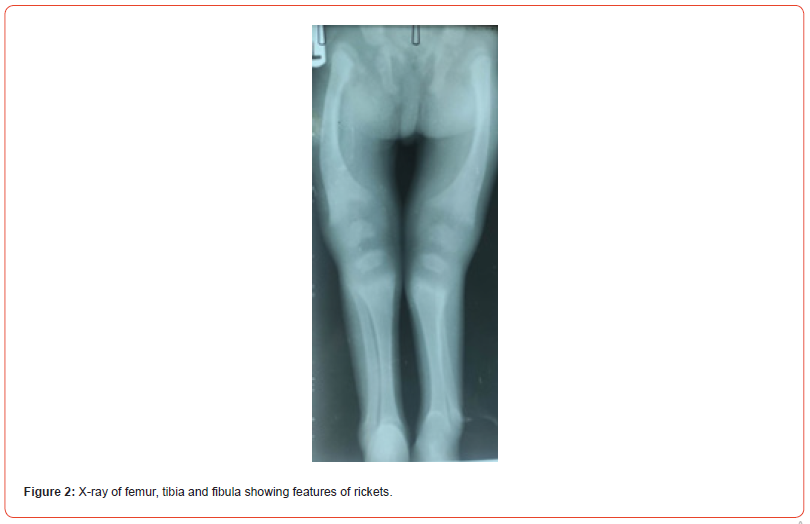
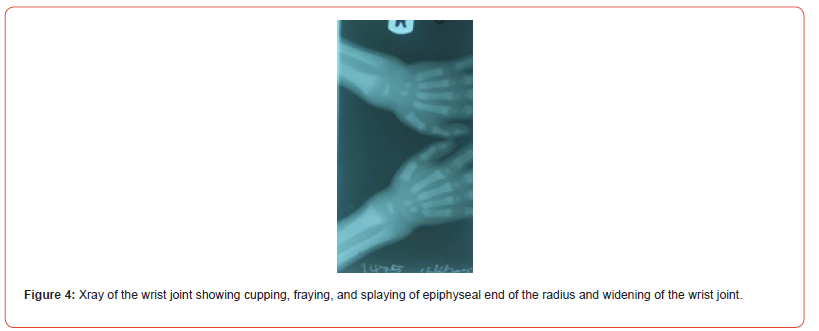
Assessment
a) CKD: mineral bone disease- secondary to right polycystic
kidney disease and absent left kidney.
b) Treatment: he was admitted and commenced on intranasal
oxygen, antibiotics, and blood transfusion of sedimented
red blood cell was given at 15mls/kg, frusemide 1mg/kg, tab
calcium lactate 300mg thrice a day and tab vitamin D 400 IU
daily. Parents were counseled on the disease entity, need for
renal replacement therapy and referral to a tertiary health
institution where further investigation and interventions would
be made available. The patient was taken away by parents and
died at home two days later.
Discussion
Congenital anomalies of the kidney and urinary tract (CAKUT) remain a major cause of chronic kidney disease (CKD) in children3. In index patients, there was delay in diagnosis of the congenital anomaly until he was eight years old when he had features of chronic kidney diseases. The anomaly could have been diagnosed while in-utero with antenatal abdominopelvic ultrasound, subsequently, counseling and planning for renal replacement therapy would have been done earlier. The true burden of CAKUT may not be known in the developing countries because not every pregnancy is registered with appropriate antenatal care [8]. Some of these children may die in infancy and the cause of death may not be unraveled as factors such as cultural/religious belief, fear of disfigurement and emotional stress on parents usually do not favour the conventional postmortem in this society [9]. The ones that survive infancy period may end up presenting with features of CKD as seen in index patient.
The features of short stature and bony deformity were noticed when he started walking. There was a developmental delay as the patient did not walk until the second year of life. This could have been accounted for by the on-going demineralization of the bone due to renal insufficiency. The mineral bone disease of CKD usually affects the normal bone turnover and mineralization processes, resulting in reduced bone strength, bone pain, fractures, poor final height attainment and subsequent short stature [10-15]. These demineralization processes start in the early stages of CKD and worsen as CKD progresses [16]. The kidneys play major roles in production of the active form of Vitamin D- 1,25 dihydroxyl cholecalciferol (1,25 DHCC). Vitamin D undergoes hydroxylation in the liver to 25-hydroxyl vitamin D, which is a biologically inactive form. There is 1α hydroxylation of 25- hydroxyl Vitamin D in the kidneys to form the active 1,25 DHCC (Calcitriol) which increases intestinal absorption of calcium and phosphates, hereby promoting bone formation [17]. This function is impaired when there is an abnormality in the development of the kidneys. This may explain the hypocalcemia in this patient. The extent of bone mineralization is dependent on calcium availability as well as on the extracellular levels of phosphate and pyrophosphate which is controlled by alkaline phosphatase enzymes [18].
Bone alkaline phosphatase may increase local phosphorus concentrations. The phosphate level is expected to be on the high side but the result from the laboratory showed it to be within normal limit. The rise in alkaline phosphatase in index patient demonstrates high turnover rate in attempt at new bone formation with increase potential for a rise in the serum phosphate. However, it has been noted that biomarkers such as calcium, phosphate and alkaline phosphatase may not correspond well in quantifying bone mineralization in the general population and in disease states such as osteoporosis or CKD. These further buttresses the fact that other factors such as FGF 23 play a key role in bone demineralization as earlier suggested [19]. In CKD stages 2–3, levels of FGF23 rise in order to enhance urinary phosphate excretion. These increased values also suppress renal 1-alpha hydroxylase, resulting in reduced 1,25(OH)_2 vitamin D (calcitriol, 1,25(OH)_2 D) values and, subsequently, increased PTH levels, which also help to reduce blood phosphate levels. In advanced CKD, however, rising PTH levels cause a release of calcium and phosphorus from bone and, when compensatory mechanisms fail, severely impaired glomerular filtration rate (GFR) results in phosphate retention which itself directly suppresses 1α-hydroxylase activity [20]. The parathyroid hormonal assay was not done due to financial constraint. It is expected that there would be secondary hyperparathyroidism from the hypocalcemia as the body fights to keep the calcium level in the normal range.
Features of chronic kidney disease (CKD) such as anaemia were seen in this patient. The haematocrit was 14.9%. The cause of anaemia in CKD is multifaceted; kidneys are responsible majorly for production of erythropoietin that drives new red blood cell formation in the bone marrow. Dysgenesis of the kidney includes aplasia, dysplasia, hypoplasia, and cystic disease. In the patient, there was absence of the right kidney with polycystic left kidney. Unilateral renal agenesis is a congenital anomaly that occurs in 1 in 3000 births [21]. Some individuals are diagnosed as having unilateral renal agenesis based on the finding of an absent kidney on ultrasonography or excretory urography. The coexistence of polycystic kidney (autosomal dominant polycystic kidney disease) and contralateral renal agenesis is rare and occurs in approximately 1 in 1 500 000-3 000 000 individuals [22]. A renal computed tomography would have given detailed images of the renal outline; however, the parents could not afford the high cost of such investigation especially in patients making “out of pocket” payment with financial constraint. The specific genetic abnormality in index patient could not be determined because of the absence of the facility for genetic study in our institution. In spite of these limitations, there is a need to have a high index of suspicion of underlying renal disease in patients with bone deformities. The fact that the child survived for 8 years suggested that he probably had some remnant renal parenchyma that was begging to be helped with renal replacement therapy until this thinned out.
In developing countries with limited resources and dwindling number of personnel due to poor economy and brain drain, prevention is the ultimate weapon. It is imperative to create awareness of the existence of CAKUT in the developing countries and to insist on timely antenatal ultrasound for early diagnosis and planned intervention. The index patient was taken home by the parent and did not go to the tertiary referral hospital. This typifies the fate of most children with chronic illness in the developing countries as parents/guardians may not be able to afford out of pocket payment for the health bill and the health insurance scheme does not have wide coverage. There are more causes of preventable mortality in children that will emerge as advances in diagnostic tools and clinical acumen unfold. Non-nutritional rickets may mirror a chronic kidney disease in children.
References
- Moe S, Drueke T, Cunningham J, Goodman W, Martin K, et al. (2006) Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: improving Global Outcomes (KDIGO). Kidney Int 69(11): 1945-1953.
- National Kidney Foundation (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39(2 Suppl 1): S1-S266.
- Asinobi AO, Ademoila AD, Ogunjunle OO, Mott SA (2014) Paediatric end-stage renal disease in a tertiary hospital in South West Nigeria. BMC Nephrol 15: 25.
- Ibadin OM, Ofovwe EG (2004) Chronic renal failure in Children of Benin, Nigeria. Saudi J Kidney Dis Transplant 15(1): 79-83.
- Odetunde OI, Okafor HU, Uwaezuoke SN, Ezeonwu BU, Adiele KD, et al. (2014) Chronic kidney disease in children as seen in a tertiary hospital in Enugu, South East, Nigeria. Niger J Clin Pract 17: 196-200.
- Anochie I, Eke F (2003) Chronic renal failure in children: a report from Port Harcourt, Nigeria (1985-2000). Pediatr Nephrol 18(7): 692-695.
- Olowu WA, Adefehinti O, Aladekomo TA (2013) Epidemiology and clinicopathologic outcome of pediatric chronic kidney disease in Nigeria, a single center study. Arab J Nephrol Transplant 6(2): 105-113.
- Nylander PP, Adekunle AO (1990) Antenatal care in developing countries. Baillieres Clin Obstet Gynaecol 4(1): 169-186.
- Sreenivas A, Jaihind Jothikaran TA, Lewis L, Mathew M (2023) Community perceptions of postmortem examination and minimally invasive tissue sampling in neonates: a qualitative study in South India. BMC Pregnancy Childbirth 23(1): 804.
- Lalayiannis AD, Soeiro EMD, Moysés RMA, Shroff R (2024) Chronic kidney disease mineral bone disorder in childhood and young adulthood: a 'growing' understanding. Pediatr Nephrol 39(3): 723-739.
- Groothoff JW, Offringa M, Van Eck-Smit BL, Gruppen MP, Nicole JK, et al. (2003) Severe bone disease and low bone mineral density after juvenile renal failure. Kidney Int 63(1): 266-275.
- Denburg MR, Tsampalieros AK, Boer IH, Kalkwarf HJ, Zemel BS, et al. (2013) Mineral metabolism and cortical volumetric bone mineral density in childhood chronic kidney disease. J Clin Endocrinol Metab 98(5): 1930-1938.
- Groothoff JW, Gruppen MP, Offringa M, Hutten J, Lilien MR, et al. (2002) Mortality and causes of death of end-stage renal disease in children: a Dutch cohort study. Kidney Int 61(2): 621-629.
- Haffner D, Schaefer F, Nissel R, Wuhl E, Tonshoff B, et al. (2000) Effect of growth hormone treatment on the adult height of children with chronic renal failure. German Study Group for Growth Hormone Treatment in Chronic Renal Failure. N Engl J Med 343(13): 923-930.
- Borzych D, Rees L, Ha IS, Chua A, Valles PG, et al. (2010) The bone and mineral disorder of children undergoing chronic peritoneal dialysis. Kidney Int 78(12): 1295-1304.
- Wesseling-Perry K (2015) Defective skeletal mineralization in pediatric CKD. Curr Osteoporos Rep 13(2): 98-105.
- Dusso AS, Brown AJ, Slatopolsky E (2005) Vitamin D. Am J Physiol Renal Physiol 289(1): F8-28.
- Clarke B (2008) Normal bone anatomy and physiology. Clin J Am Soc Nephrol 3: S131-139.
- Katherine Wesseling-Perry, Isidro B. Salusky (2013) Chronic Kidney Disease: Mineral and Bone Disorder in Children. Semin Nephrol 33(2): 169-179.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1-S130.
- Wilson RD, Baird PA (1985) Renal agenesis in British Columbia. Am J Med Genet 21(1): 153-169.
- Ramón P, Cristina A, Ana A, Rosa Zometaa, Claudia Tapiaa, et al. (2012) Autosomal dominant polycystic kidney disease with contralateral renal agenesis. Nefrologia 32(6): 839-842.
-
Adebukola Ajite*, Oludare Oluwayemi, Boluwatife Oniyide and Samuel Adisa. CKD-Mineral Bone Disease, A Feature of Delayed Diagnosis of Congenital Anomaly of The Kidney and Urinary Tract in An Eight-Year-Old Nigerian Boy- Case Report. Annals of Urology & Nephrology. 4(3): 2024. AUN.MS.ID.000589.
-
CKD-mineral bone disease; end stage renal disease; congenital anomaly of the kidneys and the urinary tract; renal replacement therapy; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






